By Kristin Higgins, MD, Medical Director of Radiation Oncology, Winship Cancer Institute of Emory University
In April 2020, a healthy, active 51-year-old woman scheduled for knee replacement at her local hospital in South Georgia underwent a routine pre-op chest X-ray, which showed a suspicious mass in her lung. A follow-up CT revealed a 2.5 cm mass in the upper left lobe. Biopsy found small cell lung cancer (SCLC). The woman self-referred to Winship Cancer Institute of Emory University, knowing its experience with this aggressive and relatively uncommon disease, which represents only 10-15 percent of all lung cancers.
At Winship, the lung cancer team ordered a PET scan and brain MRI. A PET scan and brain MRI showed the cancer had almost doubled since her diagnosis two months earlier but had not spread outside the left lung. Because the cancer was still in the limited stage (where cure is considered possible), we believed she was an excellent candidate for a clinical trial underway at Winship. Emory’s multidisciplinary thoracic tumor board reviewed her case and agreed. The patient also agreed and entered the trial in May.
Funded by the National Cancer Institute, this national trial is led by Winship, headed by Dr. Kirstin Higgins. It compares standard of care chemoradiation for SCLC with the same treatment plus immunotherapy with atezolizumab. When the monoclonal antibody atezolizumab received FDA approval in 2019, it was the first new therapy of any kind approved for SCLC in decades. However, approval for use in SCLC outside of clinical trials was only for extensive stage disease. This trial is designed to determine if it has an impact at an earlier stage.
The patient was randomly assigned to the control arm, meaning standard treatment without immunotherapy. Some patients find this disappointing; she simply said she knew she would get the best care possible.
In early June, she had the first of four cycles of platinum chemotherapy that she tolerated well.
The tumor response was immediate. CT mapping, performed the third week of June in preparation for radiation to begin three weeks after the first chemotherapy session, showed that the tumor already had shrunk from 5.2 cm (on her first CT scan at Winship) to 2 cm.
Radiation consisted of 45 Gy delivered twice daily for three weeks. Weekly CT scans showed the tumor continuing to shrink. Again, the patient experienced no side effects.
At the end of July, she completed radiation and all four chemo cycles. She resumed work and activities of daily life. She returns to Winship at Emory for a CT scan every three months for the next two years, every six months for the following five years, then yearly after that. Meanwhile, clinical trial enrollment has continued to rise at Winship and nationally.
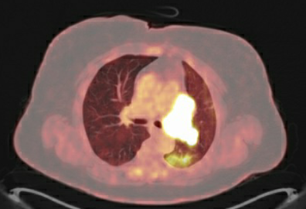
Baseline PET image
(no treatment yet)
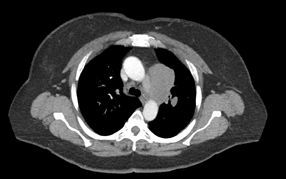
Baseline CT image
(no treatment yet)
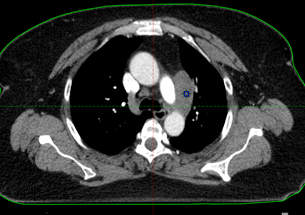
CT after first cycle of chemo
(used for radiation mapping)
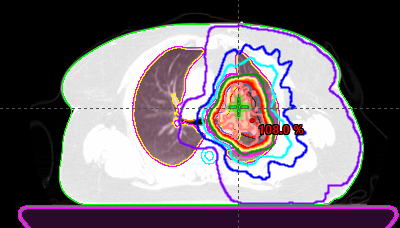
Image of radiation plan
This case illustrates two important things. First, clinical trials provide new hope for a disease for which there have been fewer advances compared to other lung cancers. Immunotherapy has not been widely investigated for SCLC, in part because it lacks the genetic markers that serve as immunotherapy targets in non-small cell lung cancer. However, many researchers believe immunotherapy holds promise in enhancing and extending response to chemoradiation. Winship’s extensive research program in SCLC includes seven clinical trials, five focusing on immunotherapy.
Second, the case shows that despite the challenges of COVID-19, clinical care and research are proceeding full-speed at Winship. Many centers have closed clinical trials. Winship has not, believing the search for more powerful treatments can’t wait. It also was important that this woman’s local physician did not delay her CT and biopsy due to the pandemic, thus making possible her early diagnosis.
The patient also sought care quickly and confidently, knowing Emory Healthcare’s safety plans were developed by the infectious disease experts who successfully fought Ebola without exposure to themselves or others. Plans include thorough disinfection, COVID-19 screenings, temperature checks, universal masking for patients and staff and delineated social distancing. Her cancer could not wait and Emory Healthcare was there for her.
Small cell lung cancer patient Lisa Leich was diagnosed after the COVID-19 pandemic hit but she got into a clinical trial at Winship right away and is getting treated before her cancer spreads. Dr. Kristin Higgins explains why this trial is unique.
To find clinical trials at Emory’s Winship Cancer Institute, visit emoryhealthcare.org/referpatient or call 1-888-WINSHIP.
Winship Cancer Institute of Emory University is committed to the continued health and safety of all patients. During this time, we are taking all necessary precautions to screen for COVID-19 and to prevent its potential spread. We continue to monitor the evolving COVID-19 situation and are working with experts throughout Emory Healthcare to keep your patients safe. For the most up-to-date information for our referring partners, click here.
Winship Physician Directory
Exclusively for physicians and advanced practice providers, find Winship at Emory physicians by name, location or treatment specialty and gain access to direct contact information. Access the WinshipMD App at: winshipcancer.emory.edu/mddirectory

